The intensification of production systems and the expansion of monocultures make agricultural systems more vulnerable to varietal resistance breakdown. Once adapted to a new resistant variety, a pathogen benefits from a large area of identical plants in which to spread rapidly.
A common scenario involves cycles of expansion in the use of a resistant variety, followed by sharp decline when it becomes susceptible due to pathogen adaptation. These cycles are often called “boom and bust.”
Although this deployment strategy may work temporarily, it is not sustainable if resistance factors are limited and non-renewable resources. Unfortunately, for a given pathogen and crop, only a few resistance genes are usually identified.
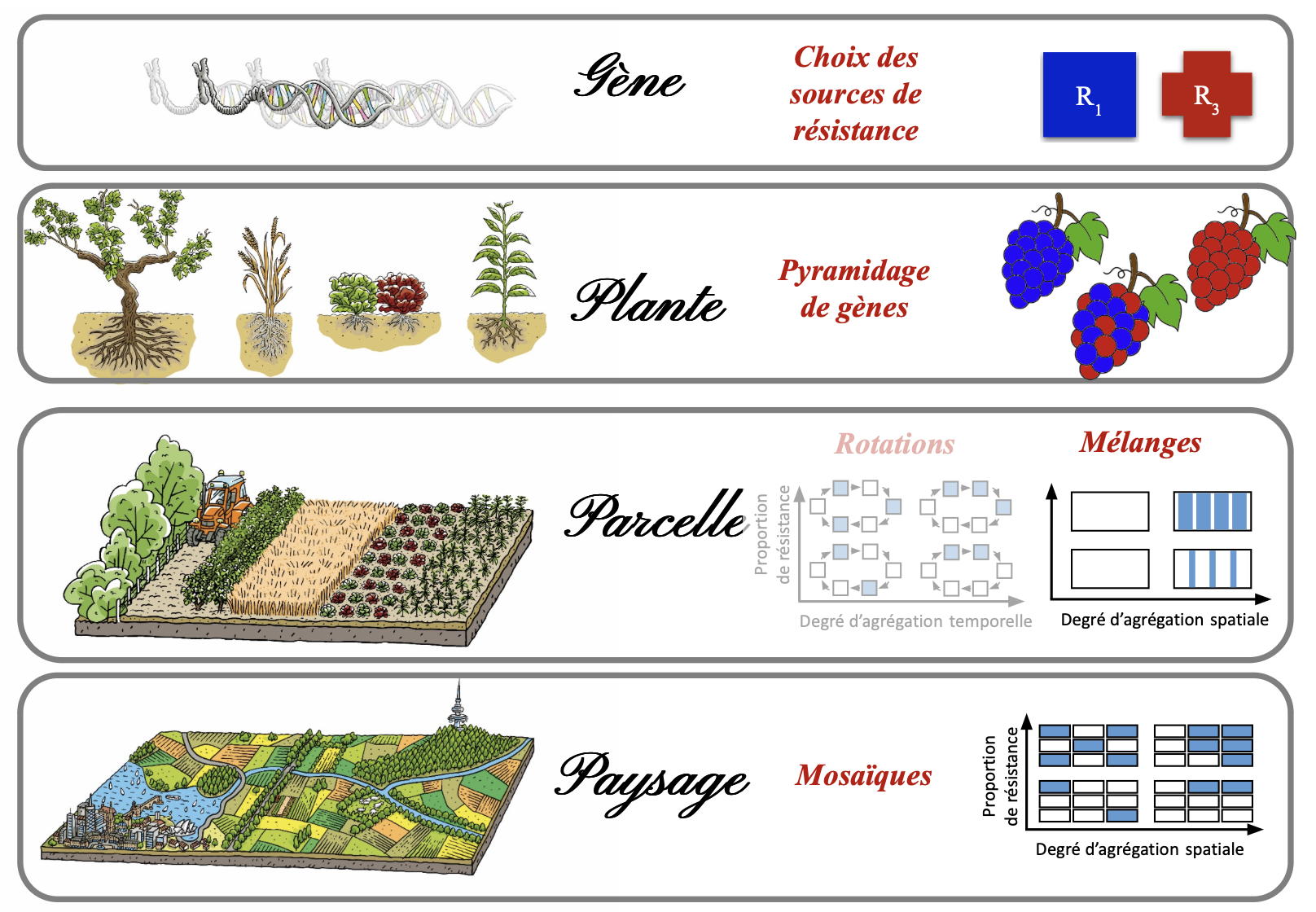

Developing more sustainable deployment strategies involves diversifying sources of resistance over time and space, at scales ranging from the plant to the landscape. The dual objective is to control pathogens to reduce crop losses (epidemiological control) while delaying or preventing resistance breakdown (evolutionary control).
Some of these approaches are already in use (pyramiding, varietal mixing, etc.), while others—drawn from research on sustainable crop protection systems—remain largely theoretical (landscape mosaics). By combining them, a wide range of strategies becomes available.
Many resistance factors identified in wild plants are not relevant or easy to use in breeding programs due to either low protective effect or insufficient introgression level. For grapevine, among the roughly thirty known resistances to downy mildew, only four are actually used in registered varieties.
Resistances are generally classified by their effectiveness (total or partial), genetic basis (mono- or polygenic), and spectrum of action (specific or non-specific to pathogen strains). Qualitative resistances are active only against certain pathogen strains. They are usually mono- or oligogenic and total, as they block disease development. Quantitative resistances are generally active against all strains (non-specific). They are most often polygenic, with a quantitative nature reflected in slowing rather than blocking pathogen development. It is crucial not to confuse these three characteristics through overgeneralization. For example, grapevine resistances to downy mildew are quantitative resistances with monogenic inheritance.
Many resistance factors identified in wild plants are not relevant or easy to use in breeding programs due to either low protective effect or insufficient introgression level. For grapevine, among the roughly thirty known resistances to downy mildew, only four are actually used in registered varieties.
Resistances are generally classified by their effectiveness (total or partial), genetic basis (mono- or polygenic), and spectrum of action (specific or non-specific to pathogen strains). Qualitative resistances are active only against certain pathogen strains. They are usually mono- or oligogenic and total, as they block disease development. Quantitative resistances are generally active against all strains (non-specific). They are most often polygenic, with a quantitative nature reflected in slowing rather than blocking pathogen development. It is crucial not to confuse these three characteristics through overgeneralization. For example, grapevine resistances to downy mildew are quantitative resistances with monogenic inheritance.
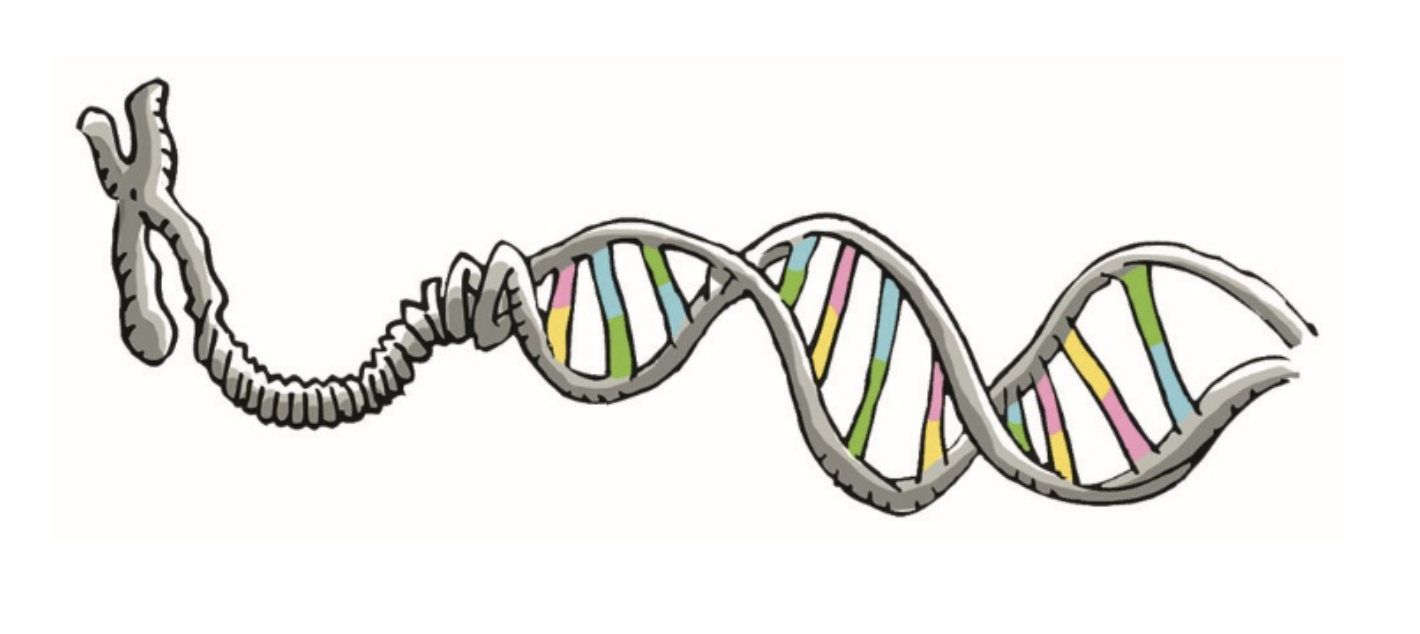
Gene pyramiding involves selecting varieties carrying multiple resistance genes. This practice, akin to diversifying resistances at the individual plant level, aims to delay the initial step of resistance breakdown by reducing the likelihood that a pathogen emerges (from a susceptible plant) with all the genetic mutations needed to overcome the pyramided genes. Indeed, the required mutations are more numerous and each potentially imposes a fitness cost, reducing the competitiveness of virulent mutants compared to strains infecting susceptible plants.
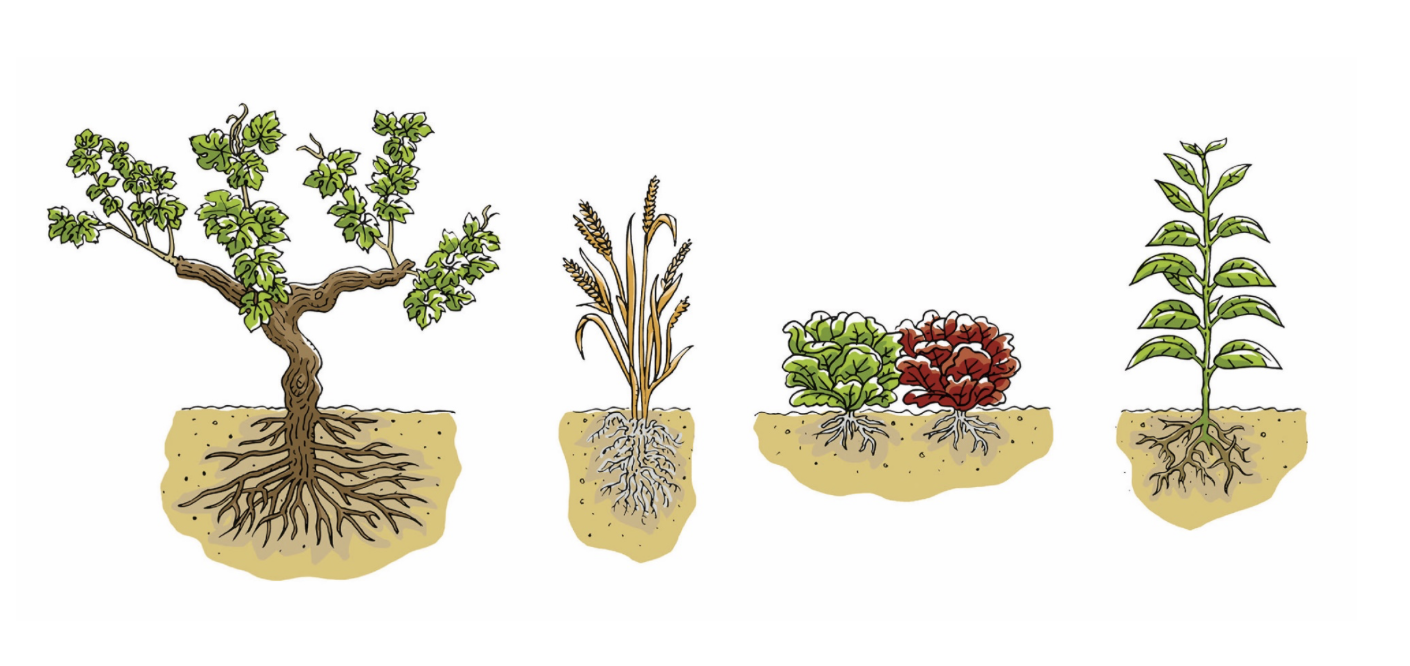
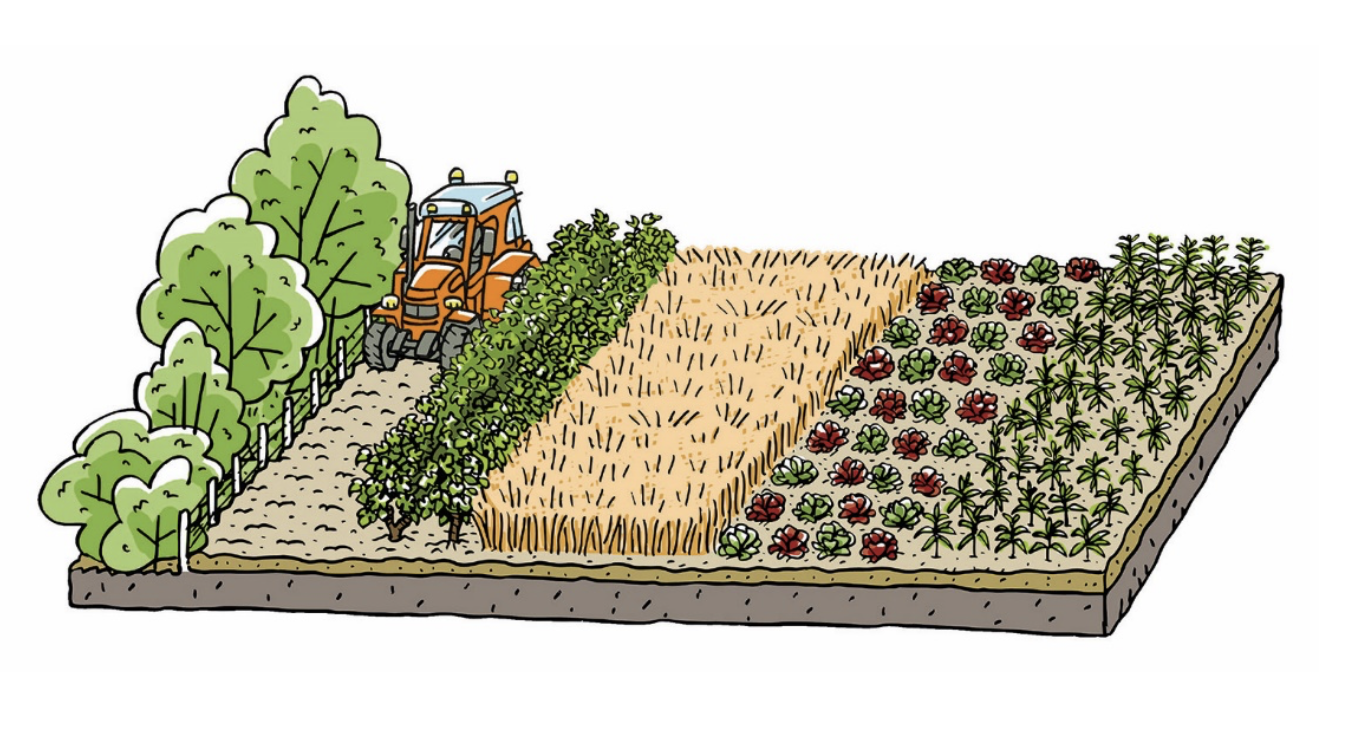
To diversify resistances within their fields, farmers can plant mixtures of crop varieties by combining completely different varieties (classic varietal mixtures), using “population” varieties (genetically unfixed), or multilines (lines differing only by their resistance genes). Crop associations are also an option.
All these approaches involve cultivating plants with diverse susceptibilities to a disease in the same field. They differ in the degree of genetic heterogeneity among the mixture components and in the planting pattern, which results in more or less aggregation of plants of the same variety.
To diversify resistances within their fields, farmers can plant mixtures of crop varieties by combining completely different varieties (classic varietal mixtures), using “population” varieties (genetically unfixed), or multilines (lines differing only by their resistance genes). Crop associations are also an option.
All these approaches involve cultivating plants with diverse susceptibilities to a disease in the same field. They differ in the degree of genetic heterogeneity among the mixture components and in the planting pattern, which results in more or less aggregation of plants of the same variety.
Cultivated diversity can also be distributed not within a single field but among fields in a landscape, known as landscape mosaics. These strategies act on resistance breakdown by limiting the establishment and spread of virulent pathogens through several mechanisms: reducing the density of a given variety, dilution effect caused by increased distances between plants of the same variety, and selecting strains that are either generalists (able to infect all varieties but generally less aggressive) or specialists (well adapted to one variety but not others).

Each choice made by breeders (gene selection, pyramiding) and farmers (mixtures, mosaics, number of components, relative proportions, aggregation level, etc.) can differently impact pathogen spread and evolution, as well as the practical feasibility of the selection scheme.
The total number of possible combinations becomes rapidly enormous. Given this vast possibility space, the large scale involved (typically a production basin), and the time needed to observe pathogen evolution (usually several years), experimentation alone is ineffective to test and compare comprehensive deployment strategies.
In this context, it is necessary both to rely on mathematical modeling and to collectively enhance field monitoring. The OSCAR observatory fully contributes to achieving this objective.
